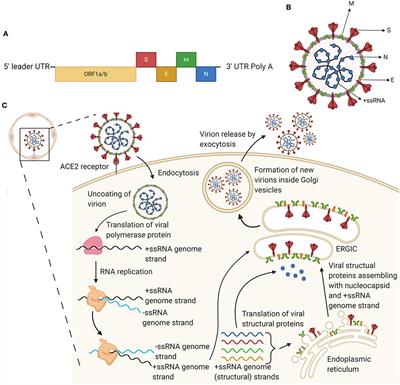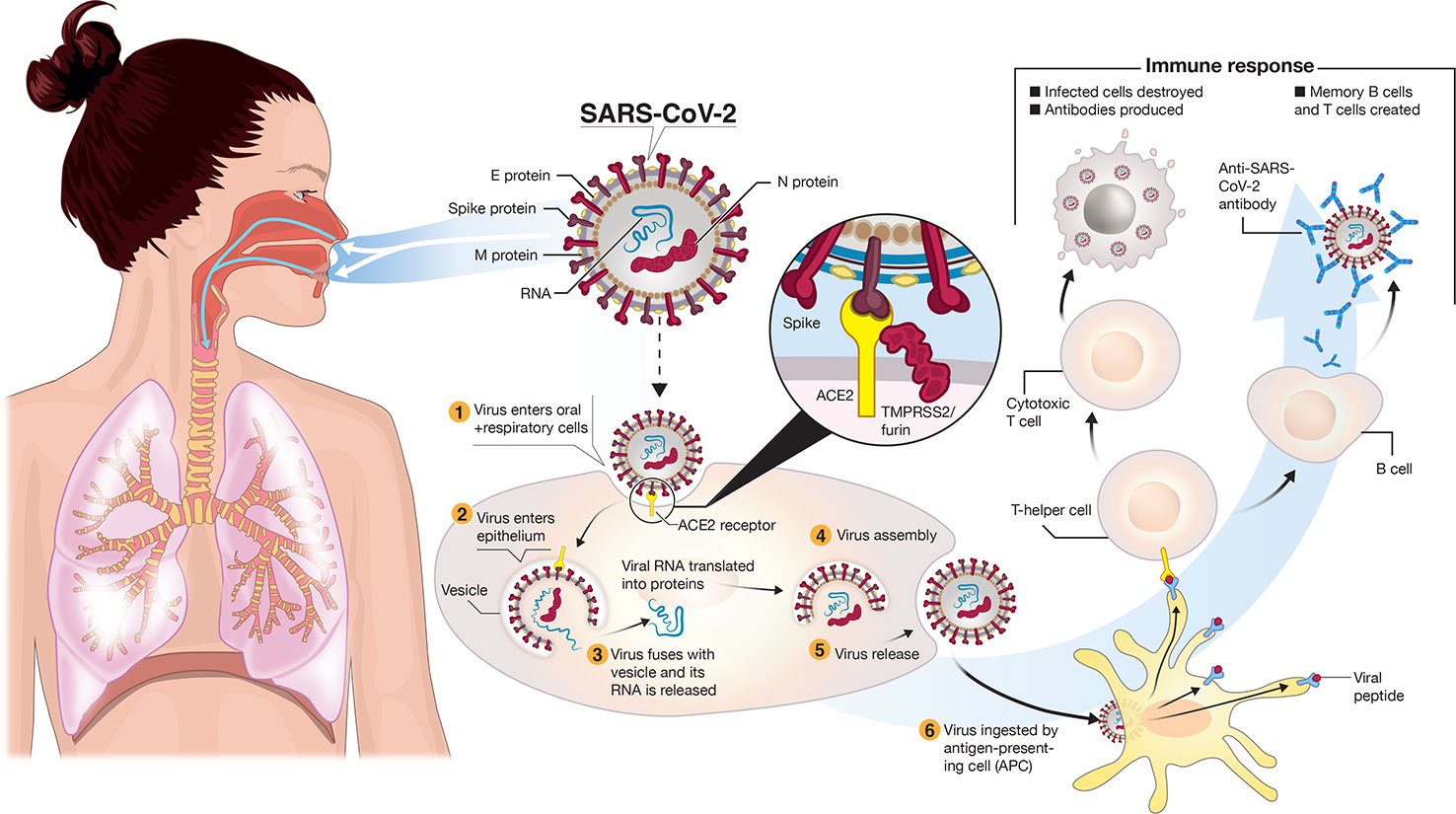
Frontiers | A Snapshot of the Global Race for Vaccines Targeting SARS-CoV-2 and the COVID-19 Pandemic
Johnson & Johnson (Janssen Pharmaceutical Cos.) - Single-Shot COVID-19 Vaccine (formerly JNJ-78436735, Ad26.COV2.S)

Johnson & Johnson Announces European Commission Approval for Janssen's Preventive Ebola Vaccine | Johnson & Johnson

Johnson & Johnson Announces Single-Shot Janssen COVID-19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of its Phase 3 ENSEMBLE Trial

University of Chicago Medicine begins recruiting participants for phase 3 COVID-19 investigational vaccine clinical trial - UChicago Medicine

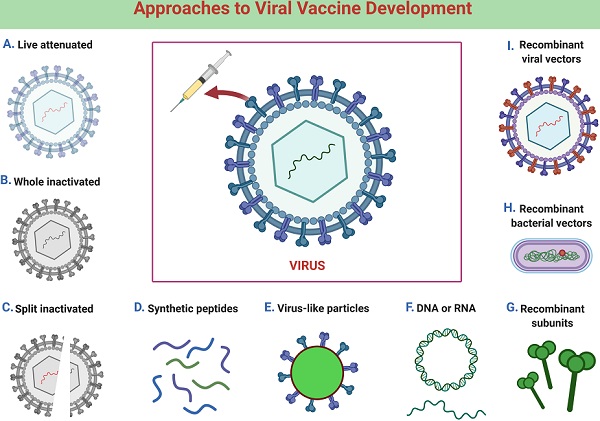
SARS-CoV-2 Vaccine Development: An Overview and Perspectives | ACS Pharmacology & Translational Science
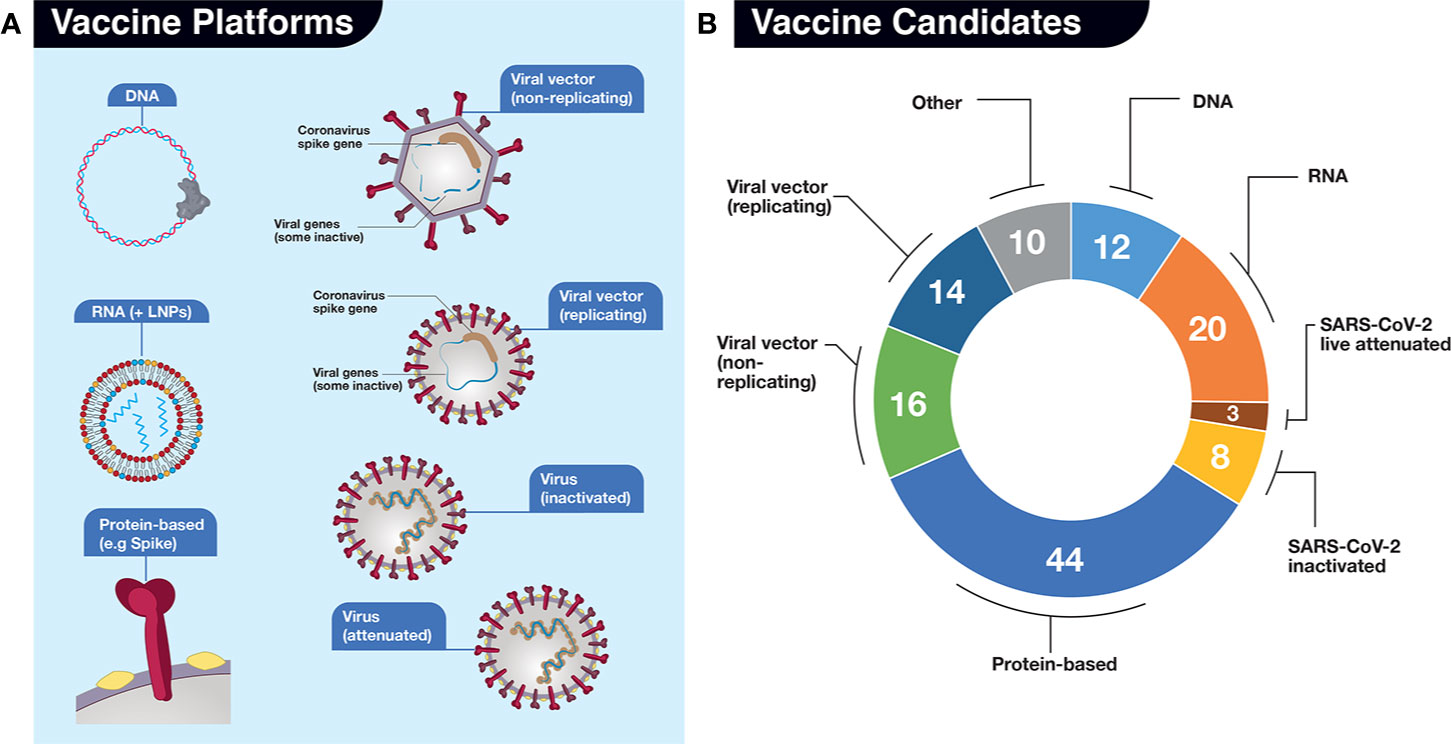
Frontiers | A Snapshot of the Global Race for Vaccines Targeting SARS-CoV-2 and the COVID-19 Pandemic
Vaccines | Free Full-Text | COVID-19 Vaccines (Revisited) and Oral-Mucosal Vector System as a Potential Vaccine Platform
P00081 Modification to MCDC OTA No. W150KN-16-9-1002 BETWEEN JANSSEN PHARMACEUTICALS, INC. 1125 TRENTON-HARBOURTON ROAD TITUSVIL