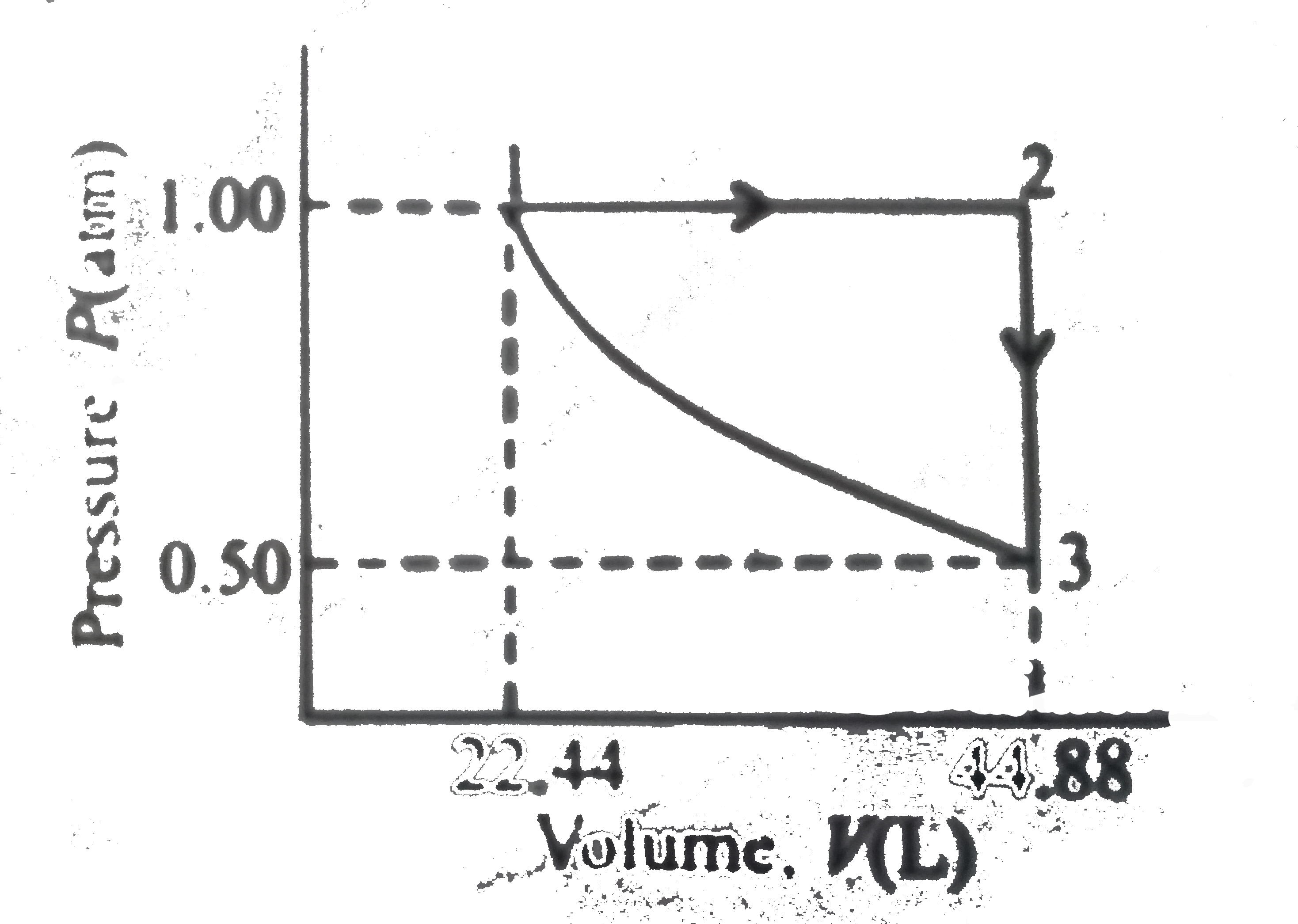
A sample consisting of 1mol of a mono-atomic perfect gas (C(V) = (3)/(2)R) is taken through the cycle as shown. Temperature at points (1),(2) and (3) respectively is

CV curves in 1.0 mol L −1 ethanol and 1.0 mol L −1 KOH with a sweep... | Download Scientific Diagram

n-moles of an ideal gas with constant volume heat capacity CV undergo an isobaric expansion - YouTube

Calculations in Chemistry To calculate the number of moles in a solid we use the following Mole Triangle g n gfm g = Mass in Grams n= Number of moles. - ppt download
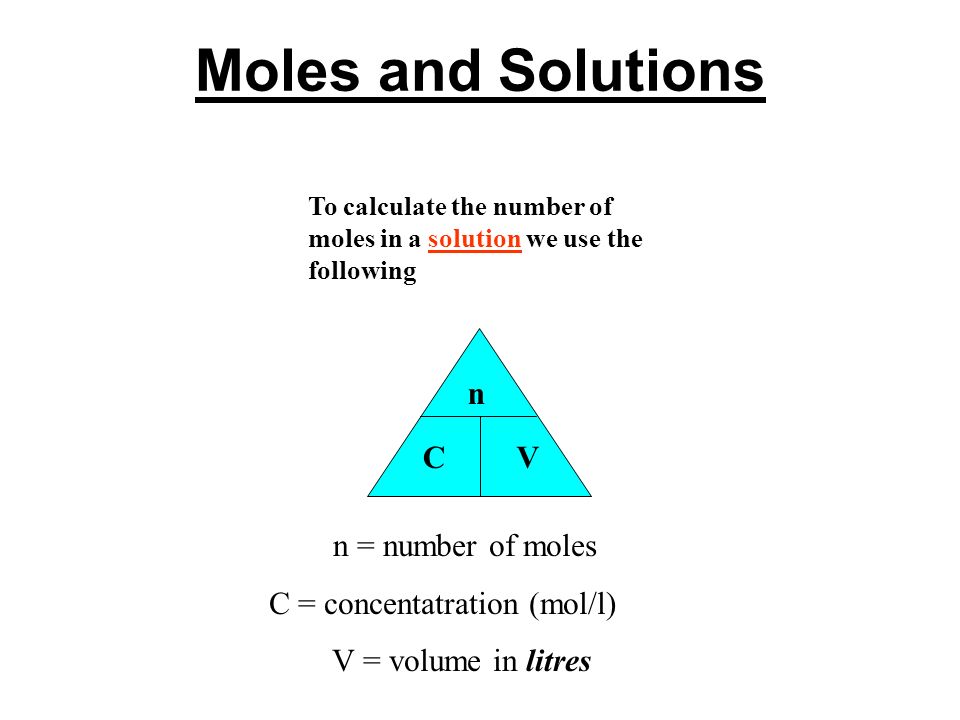
Moles and Solutions g n gfm To calculate the number of moles in a solution we use the following n CV n = number of moles C = concentatration (mol/l) V. - ppt download

SOLVED: If 2.50 mol of He gas with CV 1.5R nearly independent of T goes from 25°C and 1.00 bar to 60°C and 2.00 bar, find whichever of the following quantities can

Calculations in Chemistry To calculate the number of moles in a solid we use the following Mole Triangle g n gfm g = Mass in Grams n= Number of moles. - ppt download




![N=CV, CONCENTRATION, VOLUME, NUMBER OF MOLES [Last minute revision] | Chemistry at glance - YouTube N=CV, CONCENTRATION, VOLUME, NUMBER OF MOLES [Last minute revision] | Chemistry at glance - YouTube](https://i.ytimg.com/vi/rh4IuW1fP6g/hqdefault.jpg)
